http://slideplayer.com/slide/4206817/
chemistry
Tuesday, May 10, 2016
The ideal gas law
An ideal gas is modeled on the kinetic theory of gases which has 4 basic postulates: gases consist of small particles which are in continuous random motion, the volume of the molecules present is negligible compared to the total volume occupied by the gas, intermolecular forces are negligible, pressure is due to the gas molecules colliding with the walls of the container. The mathematical equation used to represent the ideal gas law is PV=nRT

http://slideplayer.com/slide/4206817/
http://slideplayer.com/slide/4206817/
Charles' Law
Charles' Law tells us that temperature and volume vary directly with each other. This holds true at constant pressure. Temperature for all Charles' Law problems must be in Kelvin. As the temperature of a gas increases, it gains energy. This will result in an increase in contacting the sides of their containers they are going to want to expand. The mathematical formula that represents this relationship is V1/T1=V2/T2.

http://agaul01.blogspot.com/2014/04/boyles-charles-law-in-relation-to.html
Here are some helpful links
http://www.sparknotes.com/testprep/books/sat2/chemistry/chapter5section8.rhtml
http://www.iun.edu/~cpanhd/C101webnotes/gases/charleslaw.html

http://agaul01.blogspot.com/2014/04/boyles-charles-law-in-relation-to.html
Here are some helpful links
http://www.sparknotes.com/testprep/books/sat2/chemistry/chapter5section8.rhtml
http://www.iun.edu/~cpanhd/C101webnotes/gases/charleslaw.html
Avagadro's Law
Avagadro's Law tells us that for a gas at constant temperature and pressure, the volume is directly proportional to the number of moles of gas present. Equal volumes of gases at the same temperature and pressure have the same number of particles. The mathematical formula for this relationship is V1/N1=V2/N2

http://slideplayer.com/slide/231761/
Here are some example problems

http://slideplayer.com/slide/1710660/

https://sites.google.com/site/mohshchemwithmrsp/unit-4---gas-laws/avogadro-s-law
Here are some helpful links
http://www.chemteam.info/GasLaw/Gas-Avogadro.html
http://www.britannica.com/science/Avogadros-law
https://www.youtube.com/watch?v=i-vA9uLSf7Y
http://slideplayer.com/slide/231761/
Here are some example problems
http://slideplayer.com/slide/1710660/

https://sites.google.com/site/mohshchemwithmrsp/unit-4---gas-laws/avogadro-s-law
Here are some helpful links
http://www.chemteam.info/GasLaw/Gas-Avogadro.html
http://www.britannica.com/science/Avogadros-law
https://www.youtube.com/watch?v=i-vA9uLSf7Y
Boyle's gas law
Boyle's Law tells us that the relationship between pressure and volume is an inverse relationship. It deals with two of the four factors that determine the state of a gas: pressure and volume. It holds true at at constant temperature. The mathematical equation that is used to express this relationship is P1V1=P2V2.

Here are some example problems

http://slideplayer.com/slide/2473563/

http://www.slideshare.net/makaberokurota/properties-of-matter-9977143
Here are some helpful websites
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/boyles-law
Here are some example problems
http://slideplayer.com/slide/2473563/
http://www.slideshare.net/makaberokurota/properties-of-matter-9977143
Here are some helpful websites
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/boyles-law
Monday, May 9, 2016
calculating heat
To calculate heat given the mass, specific heat and change in temperature we use the formula Q=MCdeltaT. this means that the heat in joules is equal to the mass X specific heat X change in temperature. We can use this formula to solve for any of the given variables even if it is not heat. For instance we can calculate the mass if given the heat in joules, specific heat, and change in temperature.
here are some ex problems

https://www.youtube.com/watch?v=txEDPJom6CU

https://www.youtube.com/watch?v=0jKHtBJNAYM
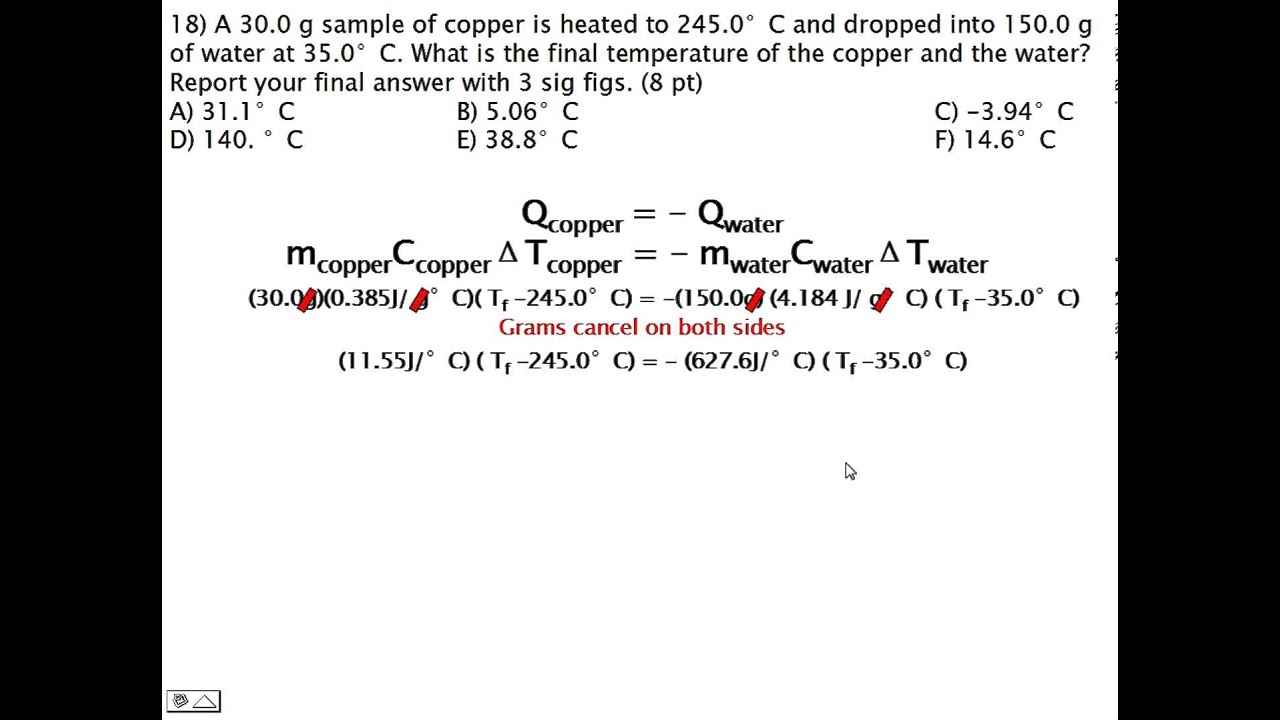
https://www.youtube.com/watch?v=EpLBZT8J3Q8
here are some more links
http://www.bbc.co.uk/schools/gcsebitesize/science/triple_aqa/calculating_energy_changes/energy_from_reactions/revision/3/
http://www.colorado.edu/physics/phys1110/phys1110_fa12/LectureNotes/Thermal.pdf
here are some ex problems

https://www.youtube.com/watch?v=txEDPJom6CU

https://www.youtube.com/watch?v=0jKHtBJNAYM

https://www.youtube.com/watch?v=EpLBZT8J3Q8
here are some more links
http://www.bbc.co.uk/schools/gcsebitesize/science/triple_aqa/calculating_energy_changes/energy_from_reactions/revision/3/
http://www.colorado.edu/physics/phys1110/phys1110_fa12/LectureNotes/Thermal.pdf
Phase cahnges and heat/cooling curves
As heat is added to a substance it undergoes some changes. Changes are changes in state which is a physical change where intermolecular bonds are broken. Melting and boiling points are determined by the vapor pressures of the solid and liquid states. At 0 degrees celsius ice and liquid water have the same vapor pressure. at 100 degrees celsius water vapor and atmospheric pressure are equal.

http://people.uwplatt.edu/~sundin/114/l114-36.htm
here are some helpful links
http://study.com/academy/lesson/what-are-heating-and-cooling-curves.html
http://www.kentchemistry.com/links/Matter/HeatingCurve.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/liquids-and-solids-11/phase-changes-90/heating-curve-for-water-394-3655/
https://www.youtube.com/watch?v=YG77v1PwQNM
http://people.uwplatt.edu/~sundin/114/l114-36.htm
here are some helpful links
http://study.com/academy/lesson/what-are-heating-and-cooling-curves.html
http://www.kentchemistry.com/links/Matter/HeatingCurve.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/liquids-and-solids-11/phase-changes-90/heating-curve-for-water-394-3655/
https://www.youtube.com/watch?v=YG77v1PwQNM
Electron dot formulas of molecules
Here are the guidelines to placing electron dot diagrams of molecules


- calculate the total number of valence electrons by adding all of the valence electrons for each atom in the molecule
- divide the total valence electrons by 2 to find the number of electron pairs in the molecule
- surround the central atom with 4 electron pair. use the remaining electron pairs to complete the octet around the other atoms
- electron pairs that are shared by atoms are called bonding electrons. the other electrons complete octets and are called non bonding electrons, or lone pairs
- if there are not enough electron pairs to provide each atom with an octet, move a non bonding electron pair between two atoms that already share an electron pair.

Subscribe to:
Comments (Atom)