On the first day of this three part lab we first added copper II chloride to a baby food jar with water. We then put a iron nail in this mixture and let it sit over night. The next day we removed the nail and washed away any of the copper on the nail using a wash bottle. Then we siphoned off the solution of Iron II Chloride. Then we added HCl and then siphoned off the acid. Lastly we washed the copper with water and siphoned off water.
Here are some pictures from lab
Tuesday, December 15, 2015
Saturday, December 12, 2015
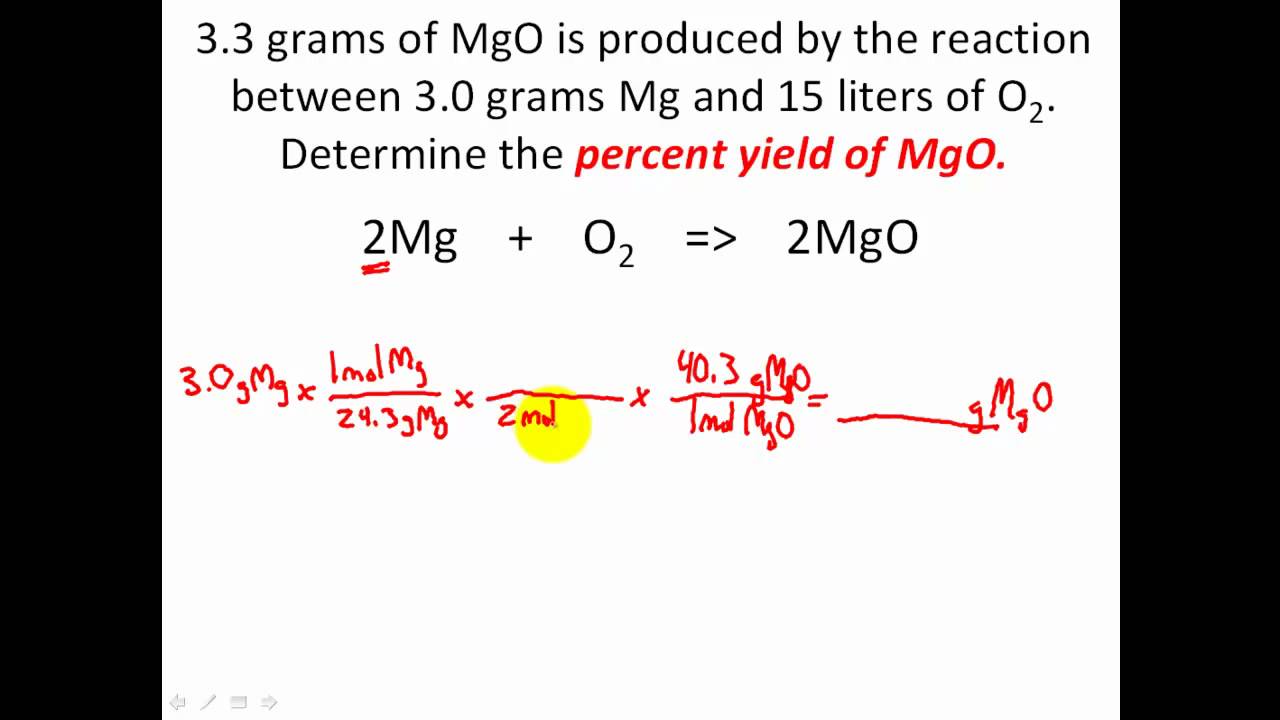
Calculating percent yield
Percent yield refers to the efficiency of a chemical reaction; defined as the actual yield/theoretical yield x100. There are many applications to this formula because it allows you to find the theoretical yield if you need it, the actual yield, or the percent yield
Here are some examples that may help you better understand these problems.
https://www.youtube.com/watch?v=0ZFUdxetdls

https://www.youtube.com/watch?v=tr9ijLSoMV4
Here are some examples that may help you better understand these problems.

https://www.youtube.com/watch?v=0ZFUdxetdls

https://www.youtube.com/watch?v=tr9ijLSoMV4
Finding the limiting reagents
The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. To find the limiting reagent in the reaction we can use two methods.
Method 1
here are some sights that may help
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Limiting_Reagents
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Method 1
- Determine the balanced chemical equation for the chemical reaction
- convert all given information into moles through use of molar mass as a conversion factor
- calculate the mole ratio from the given information. Compare the calculated ration to the actual ratio
- use the amount of limiting reactant to calculate the amount of limiting reactant to calculate the amount of product produced.
- if necessary, calculate how much is left in excess of the non-limiting reagent
- Balance the chemical equation for the chemical reaction
- convert the given information into moles
- use stoichiometry for each individual reactant to find the mass of the product produced
- the reactant that produces a lesser amount of product is the limiting reagent
- the reactant that produces a larger amount of product is the excess reagent
- to find the amount of remaining excess reactant, subtract the mass of excess reagent consumed from the total mass of excess reagent given
here are some sights that may help
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Limiting_Reagents
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Stoichiometry basics
In Greek stoikhein means element and metron means measure, so stoichiometry translates to the measure of elements. Stoichiometry involves using the relationships between reactants and/or products in a chemical reaction to determine desired quantitative data.

https://www.tes.com/lessons/ca4Wkej7zYnjWQ/solution-stoichiometry-ap-chemistry
This can help us find how many grams of product will be produced, how many grams of reactants are needed to react with another reactant to form a certain product, etc.
https://www.tes.com/lessons/ca4Wkej7zYnjWQ/solution-stoichiometry-ap-chemistry
This can help us find how many grams of product will be produced, how many grams of reactants are needed to react with another reactant to form a certain product, etc.
Thursday, December 3, 2015
Redox reactions
An redox reaction (oxidation-reduction) is a type of chemical reaction that involves a transfer of electrons between two substances. An redox reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or loosing an electron. This is the driving force of these reactions. Based on weather a substance looses or gains electrons tells you if it is the oxidizing agent, or reducing agent. To tell if the substance looses or gains electrons we use these oxidation number rules.

http://www.slideshare.net/smartensen/51-b-groups-oxidation-states
If you want more help with these reaction you can visit these sites
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
http://www.slideshare.net/smartensen/51-b-groups-oxidation-states
If you want more help with these reaction you can visit these sites
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
Acid base reactions
In acid base reactions the driving force is the production of water. These reactions form a salt and water as products. A salt can be described as a cation or base and anion of an acid. Some things that affect these reactions are the strength of the acid and base. Strong acids produce hydrogen, and their oxygen's outnumber the hydrogen's by two or more. A strong base would contain -OH, disassociate completely, and are contain group 1 and 2 metals plus the -OH.
Here are some links for some examples and practice
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
http://www.britannica.com/science/acid-base-reaction
Here are some links for some examples and practice
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
http://www.britannica.com/science/acid-base-reaction
Wednesday, December 2, 2015
Precipitation Reactions
Precipitation reactions is the formation of a solid salt when two solutions containing soluble salts are combined. Whether or not a reaction occurs can be determined using the solubility rules for ionic solids. Because not all reactions form a precipitate, you have to use the solubility rules to tell if the substance created is a insoluble salt.

https://www.youtube.com/watch?v=WPnrMTCK0aU
When looking at these reactions they can be seen in the molecular equation, complete equation, and net ionic equation as it goes through the reaction.

http://www.slideshare.net/colinquinton/04-3144335
If you want to know more about precipitation reactions you can visit these sites
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Precipitation_Reactions
https://www.youtube.com/watch?v=IIu16dy3ThI

https://www.youtube.com/watch?v=WPnrMTCK0aU
When looking at these reactions they can be seen in the molecular equation, complete equation, and net ionic equation as it goes through the reaction.
http://www.slideshare.net/colinquinton/04-3144335
If you want to know more about precipitation reactions you can visit these sites
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Precipitation_Reactions
https://www.youtube.com/watch?v=IIu16dy3ThI
Sunday, November 22, 2015
Solubility lab
In this lab we combined drops from different chemicals in a well plate and looked to see if any precipitate formed. First we gathered all of the reagents in pipettes. We then used our chart to see which of the reagents that we would be combining. When we combined the substances if no precipitate formed we put a dash through the box, but if a precipitate formed we placed its formula in the box.
Basics of chemical reactions
Chemical reactions are reactions that are usually characterized by a chemical change, and they yield one or more products. Some of the clues that a chemical reaction has occurred are color change, solid forms, bubbles form, and heat or a flame is produced. The substance or substances, initially involved in a chemical reaction are called the reactants. What the chemical reaction yields is called a product.
Here are some examples of chemical reactions

https://en.wikipedia.org/wiki/Combustion

http://www.grandinetti.org/chemical-reactions
Here are some examples of chemical reactions

https://en.wikipedia.org/wiki/Combustion
http://www.grandinetti.org/chemical-reactions
Friday, November 13, 2015
Formula of a chloride lab
This week the second lab that we performed this week was the formula of a chloride lab. First step in this lab was to take the mass of the beaker in which we would doing the experiment. Next we placed a small amount of zinc in the beaker and re-weighed. Then we got 10 ml of acid and mixed it in the beaker with the zinc. We placed the beaker on a hot plate and heated until all of the acid was gone from the substance. We let this cool for a few minutes and then re-weighed after this reaction.
Formula of a hydrate lab
In lab this week the first lab we performed was the formula of a hydrate lab. The first step in this lab was to measure the mass of the test tube in which we would be conducting the lab. Next we placed about 2 cm of hydrated crystal in the bottom of the test tube. Next we measured the new mass of the test tube with the crystals in it. We then placed the test tube on the ring stand and heated the test tube for a few minutes until the color of the substance changed from blue to white. We remeasured the mass of the test tube, and then heated the test tube again to try to drive off any left over liquid.
Sunday, November 8, 2015
Hydrate compounds
Hydrate compounds that have water molecules as part of their chemical formula. This contributes to crystalline structure of the compound.

http://www.mpbio.com/product.php?pid=02191403&country=223
In the picture above we have an example of a hydrate. The part of the formula in front of the dot is the anhydrous. The part behind the dot is the hydrate. The coefficient in front of the hydrate represents the moles present so in this compound there is one mole of water. When naming this compound we first take the hydrous, aluminum sulfate, and then put on the hydrate which is mono hydrate.
If you want to learn more about hydrates here are some helpful links
http://www.chem.purdue.edu/gchelp/nomenclature/hydrates_2009.htm
https://en.wikipedia.org/wiki/Hydrate
http://www.mpbio.com/product.php?pid=02191403&country=223
In the picture above we have an example of a hydrate. The part of the formula in front of the dot is the anhydrous. The part behind the dot is the hydrate. The coefficient in front of the hydrate represents the moles present so in this compound there is one mole of water. When naming this compound we first take the hydrous, aluminum sulfate, and then put on the hydrate which is mono hydrate.
If you want to learn more about hydrates here are some helpful links
http://www.chem.purdue.edu/gchelp/nomenclature/hydrates_2009.htm
https://en.wikipedia.org/wiki/Hydrate
Molar conversions
In converting units from representative particles to mass, we need to first convert the representative particles into moles. We use a conversion factor to convert from representative particles to moles and then from moles to mass. These conversion factors are always the same and help guide us when trying to convert into moles, mass, volume at STP, and representative particles.

https://mrdesjardinsgg12wiki.wikispaces.com/The+Mole
In the picture above it gives us an outline of how we convert things back and forth using moles. If you want to go from moles to mass, representative particles, or volume, it takes one conversion. If you want to convert volume to mass or Representative particles it takes two steps.
Here are some links that may help you learn more about the mole road map
https://www.youtube.com/watch?v=mBVL0PHPrhg
https://www.youtube.com/watch?v=zWzA-T54pPI
https://www.youtube.com/watch?v=xPdqEX_WMjo

https://mrdesjardinsgg12wiki.wikispaces.com/The+Mole
In the picture above it gives us an outline of how we convert things back and forth using moles. If you want to go from moles to mass, representative particles, or volume, it takes one conversion. If you want to convert volume to mass or Representative particles it takes two steps.
Here are some links that may help you learn more about the mole road map
https://www.youtube.com/watch?v=mBVL0PHPrhg
https://www.youtube.com/watch?v=zWzA-T54pPI
https://www.youtube.com/watch?v=xPdqEX_WMjo
Thursday, October 29, 2015
Post test reflection
Today we took the unit test over matter and measurement. The test overall was decently hard with some things that were more easy than others. I found that the parts over matter, and significant digits to be fairly easy. Were I had trouble was efficiently solving the conversion problems, as towards the end of the test I started to run out of time. Overall I thought that I should have done fairly well as I studied quite a bit the night before.
Significant figure rules
In this unit we covered a concept called significant figures. Significant figures are each of the digits of a number that are used to express it to the required degree of accuracy, starting from the first nonzero digit.

http://passyworldofmathematics.com/significant-figures/
There are several rules that go along with significant figures which can be seen above in the picture. The first rule is that all non-zero numbers are always significant. The second rule is that all zeros between non-zero numbers are always significant. The third rule is that all zeros which are simultaneously to the right of the decimal point and at the end of the number are always significant. The last rule is that all zeros which are to the left of the written decimal point are in a number greater than ten are always significant.
http://www.usca.edu/chemistry/genchem/sigfig.htm
http://passyworldofmathematics.com/significant-figures/
There are several rules that go along with significant figures which can be seen above in the picture. The first rule is that all non-zero numbers are always significant. The second rule is that all zeros between non-zero numbers are always significant. The third rule is that all zeros which are simultaneously to the right of the decimal point and at the end of the number are always significant. The last rule is that all zeros which are to the left of the written decimal point are in a number greater than ten are always significant.
| Number | # Significant Figures | Rule(s) |
| 48,923 | 5 | 1 |
| 3.967 | 4 | 1 |
| 900.06 | 5 | 1,2,4 |
| 0.0004 (= 4 E-4) | 1 | 1,4 |
| 8.1000 | 5 | 1,3 |
| 501.040 | 6 | 1,2,3,4 |
| 3,000,000 (= 3 E+6) | 1 | 1 |
| 10.0 (= 1.00 E+1) | 3 | 1,3,4 |
Here are some helpful links to learn more
Sunday, October 25, 2015
Matter
In our first section of measurement we covered matter. Matter is anything that has mass and takes up space. There are three states in which we find matter, which are water, liquid, and gas. We classify this matter using a chart with many levels. if the matter is uniform it is homogeneous. If the matter is not then it is heterogeneous. If the matter was homogeneous and has a variable composition it is a homogeneous mixture, but if it doesn't have a variable composition then it is a pure substance. If the matter is a pure substance and can be separated into simple substances it is a compound, and if not it is an element. With matter there are physical properties which are observed without changing into another substance, and there are chemical properties which can only be observed when changing into another substance. Some physical properties are boiling point,and density. Some chemical properties are heat of combustion, chemical stability, and toxicity.

http://sciencewithme.com/learn-about-matter/

https://www.pinterest.com/pin/478085316665314945/
here are some more helpful links about matter.
https://en.wikipedia.org/wiki/Mixture
http://www.ducksters.com/science/chemistry/chemical_mixtures.php
http://www.chem4kids.com/files/matter_states.html
http://sciencewithme.com/learn-about-matter/

https://www.pinterest.com/pin/478085316665314945/
here are some more helpful links about matter.
https://en.wikipedia.org/wiki/Mixture
http://www.ducksters.com/science/chemistry/chemical_mixtures.php
http://www.chem4kids.com/files/matter_states.html
Mole Day
On Friday we celebrated mole day in class. Mole day is an unofficial holiday celebrated among chemists on October 23, which celebrates Avogadro's number 6.02 times 10^23. To celebrate mole day we had to make a mole out of fabric and sew it together to bring into class. At first I wasn't sure how I was going to make the mole, because I didn't think I would be able to sew, but it wasn't as hard as I thought it was going to be. The first step to making the mole was to cut out the fabric to match the templates that our teacher gave us. After I had all of these cut out i started to sew along the lines as the directions stated. I then stuffed the mole, finished sewing, and then finally added eyes, nose, and a tail. I thought that this project would be fairly difficult but it turned out not to be that bad.
Thursday, October 1, 2015
Decay
In this Unit we learned about three different types of decay, alpha, beta, and gamma. Many nuclei are radioactive, which means they spontaneously decay, forming a different nucleus and producing one or more particles. The first type of decay we covered was alpha decay. Alpha decay is a common mode for heavy radioactive nuclides resulting in a loss of 4 in the mass number and a loss of 2 in atomic number.
Here is an example of Alpha decay

http://www.chemteam.info/Radioactivity/Writing-Alpha-Beta.html
The second type of decay we learned about was beta decay. Beta decay is a decay process for radioactive nuclides in which mass number does not change and the atomic number increases by 1.
Here is an example of Beta decay

http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
The third and final type of decay type of decay we learned about was Gamma decay. In gamma decay a nucleus changes from a higher energy state to a lower energy state through the emission of electromagnetic radiation but does not change the mass number, or the proton number.
Here is an example of Gamma decay

http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
If you are wishing to learn more about these types of decay you can go to these websites
Alpha-http://education.jlab.org/glossary/alphadecay.html
Beta-https://en.wikipedia.org/wiki/Beta_decay
Gamma-http://www2.lbl.gov/abc/wallchart/chapters/03/3.html
Here is an example of Alpha decay
http://www.chemteam.info/Radioactivity/Writing-Alpha-Beta.html
The second type of decay we learned about was beta decay. Beta decay is a decay process for radioactive nuclides in which mass number does not change and the atomic number increases by 1.
Here is an example of Beta decay
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
The third and final type of decay type of decay we learned about was Gamma decay. In gamma decay a nucleus changes from a higher energy state to a lower energy state through the emission of electromagnetic radiation but does not change the mass number, or the proton number.
Here is an example of Gamma decay
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
If you are wishing to learn more about these types of decay you can go to these websites
Alpha-http://education.jlab.org/glossary/alphadecay.html
Beta-https://en.wikipedia.org/wiki/Beta_decay
Gamma-http://www2.lbl.gov/abc/wallchart/chapters/03/3.html
Forensic Archaeology lab
The archaeology lab started with getting a piece of paper which had two sides. On one side of the paper it had color, and on the other it was plain white. The first step in the lab was to cut the piece of paper into 567 squares. Each of these squares would have a white side and a colored side. after we had all of the squares cut out we put them into a cup and dumped them out. we divided the squares into the ones that had the colored side face up and the white side face up.the atoms that were colored side up represented the atoms that had decayed and were taken out from the rest of the pieces. we repeated the process of dumping and removing the decayed squares for 6 times. this process represents half life of a substance as it decays.
If you want to learn more about half life visit these website
Sunday, September 27, 2015
Mass of subatomic particles
In this lesson we learned about isotopes, atomic mass and how to calculate the average atomic mass of an isotope. An isotope has the same amount of protons but a different amount of neutrons. most elements on the periodic table have multiple isotopes which is why the atomic mass on the periodic table is a weighted average. The formula we used in order to calculate the percent abundance of an isotope was the mass times the percent amount plus the mass of the other isotope to times the percent abundance of this isotope which gives you the average atomic mass.
if you want to learn more about protons, neutrons, mass, and isotopes visit these websites
http://education.jlab.org/qa/pen_number.html
https://en.wikipedia.org/wiki/Isotope
if you want to learn more about protons, neutrons, mass, and isotopes visit these websites
http://education.jlab.org/qa/pen_number.html
https://en.wikipedia.org/wiki/Isotope
Beanium Lab
In this lab we take a look at a new element discovered called Beanium. We examine the different isotopes which come in 4 different forms white, black, red, and pinto Beanium. We were given a sample of Beanium which contained these four colors of Beanium. First we counted the number of atoms of the different isotopes present. Then we added the number of atoms of each isotope together to get the total number of beanium atoms in the sample. Next we got the total mass of all the atoms of each of the different isotopes. Then we divided this number by the number of atoms of the isotope present to get the average mass of the isotope. The last piece of information we got was the percent abundance of each of the isotopes. We got this value by dividing the number of atoms of each isotope present by the total number of beanium atoms in the sample.
Thursday, September 17, 2015
Atomic theory and nuclear chemistry
The first lesson in the unit was about the different atomic theories that had existed.
The first theory was Dalton's atomic theory which had 5 postulates
- All elements are composed of atoms
- all atoms of a given element are identical
- atoms of different elements are different
- compounds consist of the atoms of different elements
- atoms are indivisible and are not created or destroyed in a chemical reaction
The law of constant composition is also covered in this section of the unit which states that a compound always contains the same proportion by mass of the element of which it is composed
We learned how to calculate the percent composition of each component in a compound because of this law of constant composition
Another person we learned about was JJ Thomson who helped to create the chocolate chip model which he discovered by using a cathode ray to show atoms of any element emit particles with a negative charge
The last theory we learned was the modern theory developed by Rutherford and his gold foil experiment which proved the presence of a positively charged center in a atom, the nucleus
Monday, September 14, 2015
reflection of the frontier chemistry project
The frontier chemistry project was a online data base that would help us to help treat the maladies given to us using the plants in the Eastern Deciduous Forest, and the Tall Grass Prairie of Missouri. We had to also go out and find 15 other plants and take pictures with them. the pictures were the hardest part of the project because it actually required me to go out and find these plants, and they were not all located in one place. it was also rather difficult to find the active chemical ingredients that was needed in the data base. after told a website that located these ingredients when given the plants name, it was fairly easy to finish off the project. after the data base was complete we had to write a essay in which we were randomly given one of the maladies and we had to treat it. this was fairly easy also as I had already done all the research necessary and could easily complete it.
Reflection of nomenclature
During the Nomenclature unit we learned how to name the different types of compounds. We learned how to take their chemical forms and tell what their name was, and take their name and tell the chemical formula. We learned binary compounds which was broken into three types; Type I, Type II and Type III. we also learned how to name acids and the polyatomic ions. It will be important to know how to know these rules of naming as they will help us in the future units to come. To practice some of these we did a Grocery store nomenclature lab which used the polyatomic ions to find items that can be found commonly in a household
Subscribe to:
Comments (Atom)











