Percent yield refers to the efficiency of a chemical reaction; defined as the actual yield/theoretical yield x100. There are many applications to this formula because it allows you to find the theoretical yield if you need it, the actual yield, or the percent yield
Here are some examples that may help you better understand these problems.
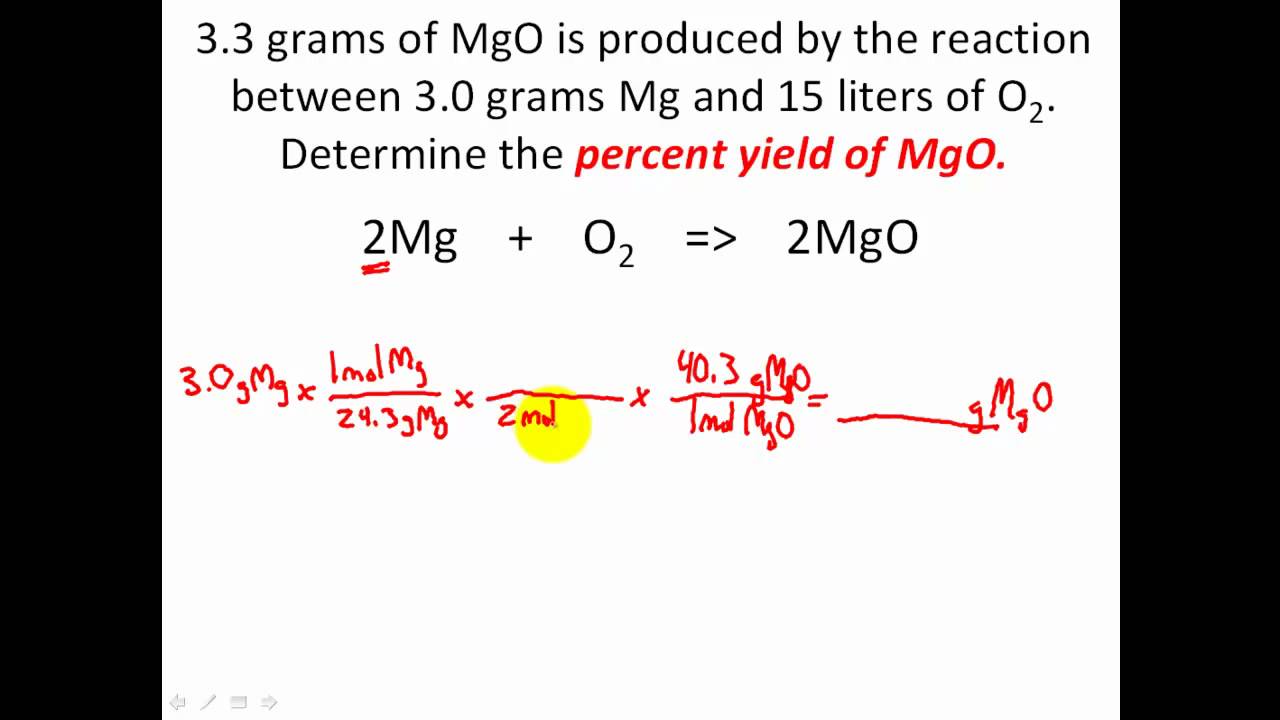 https://www.youtube.com/watch?v=0ZFUdxetdls
https://www.youtube.com/watch?v=0ZFUdxetdls
 https://www.youtube.com/watch?v=tr9ijLSoMV4
https://www.youtube.com/watch?v=tr9ijLSoMV4


Thank you for the videos! They are very helpful for studying!
ReplyDelete