On the first day of this three part lab we first added copper II chloride to a baby food jar with water. We then put a iron nail in this mixture and let it sit over night. The next day we removed the nail and washed away any of the copper on the nail using a wash bottle. Then we siphoned off the solution of Iron II Chloride. Then we added HCl and then siphoned off the acid. Lastly we washed the copper with water and siphoned off water.
Here are some pictures from lab
Tuesday, December 15, 2015
Saturday, December 12, 2015
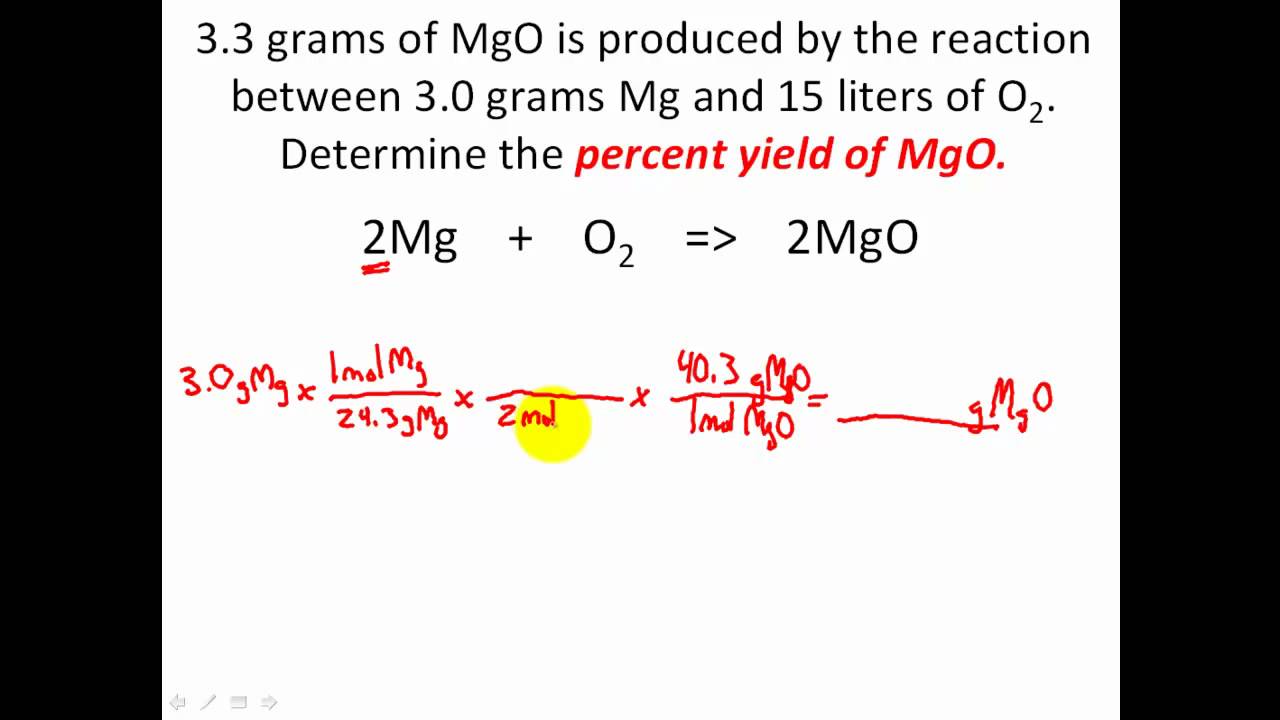
Calculating percent yield
Percent yield refers to the efficiency of a chemical reaction; defined as the actual yield/theoretical yield x100. There are many applications to this formula because it allows you to find the theoretical yield if you need it, the actual yield, or the percent yield
Here are some examples that may help you better understand these problems.
https://www.youtube.com/watch?v=0ZFUdxetdls

https://www.youtube.com/watch?v=tr9ijLSoMV4
Here are some examples that may help you better understand these problems.

https://www.youtube.com/watch?v=0ZFUdxetdls

https://www.youtube.com/watch?v=tr9ijLSoMV4
Finding the limiting reagents
The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops. To find the limiting reagent in the reaction we can use two methods.
Method 1
here are some sights that may help
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Limiting_Reagents
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Method 1
- Determine the balanced chemical equation for the chemical reaction
- convert all given information into moles through use of molar mass as a conversion factor
- calculate the mole ratio from the given information. Compare the calculated ration to the actual ratio
- use the amount of limiting reactant to calculate the amount of limiting reactant to calculate the amount of product produced.
- if necessary, calculate how much is left in excess of the non-limiting reagent
- Balance the chemical equation for the chemical reaction
- convert the given information into moles
- use stoichiometry for each individual reactant to find the mass of the product produced
- the reactant that produces a lesser amount of product is the limiting reagent
- the reactant that produces a larger amount of product is the excess reagent
- to find the amount of remaining excess reactant, subtract the mass of excess reagent consumed from the total mass of excess reagent given
here are some sights that may help
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Limiting_Reagents
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
Stoichiometry basics
In Greek stoikhein means element and metron means measure, so stoichiometry translates to the measure of elements. Stoichiometry involves using the relationships between reactants and/or products in a chemical reaction to determine desired quantitative data.

https://www.tes.com/lessons/ca4Wkej7zYnjWQ/solution-stoichiometry-ap-chemistry
This can help us find how many grams of product will be produced, how many grams of reactants are needed to react with another reactant to form a certain product, etc.

https://www.tes.com/lessons/ca4Wkej7zYnjWQ/solution-stoichiometry-ap-chemistry
This can help us find how many grams of product will be produced, how many grams of reactants are needed to react with another reactant to form a certain product, etc.
Thursday, December 3, 2015
Redox reactions
An redox reaction (oxidation-reduction) is a type of chemical reaction that involves a transfer of electrons between two substances. An redox reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or loosing an electron. This is the driving force of these reactions. Based on weather a substance looses or gains electrons tells you if it is the oxidizing agent, or reducing agent. To tell if the substance looses or gains electrons we use these oxidation number rules.

http://www.slideshare.net/smartensen/51-b-groups-oxidation-states
If you want more help with these reaction you can visit these sites
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1

http://www.slideshare.net/smartensen/51-b-groups-oxidation-states
If you want more help with these reaction you can visit these sites
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
Acid base reactions
In acid base reactions the driving force is the production of water. These reactions form a salt and water as products. A salt can be described as a cation or base and anion of an acid. Some things that affect these reactions are the strength of the acid and base. Strong acids produce hydrogen, and their oxygen's outnumber the hydrogen's by two or more. A strong base would contain -OH, disassociate completely, and are contain group 1 and 2 metals plus the -OH.
Here are some links for some examples and practice
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
http://www.britannica.com/science/acid-base-reaction
Here are some links for some examples and practice
https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidizing-and-reducing-agents-1
http://www.britannica.com/science/acid-base-reaction
Wednesday, December 2, 2015
Precipitation Reactions
Precipitation reactions is the formation of a solid salt when two solutions containing soluble salts are combined. Whether or not a reaction occurs can be determined using the solubility rules for ionic solids. Because not all reactions form a precipitate, you have to use the solubility rules to tell if the substance created is a insoluble salt.

https://www.youtube.com/watch?v=WPnrMTCK0aU
When looking at these reactions they can be seen in the molecular equation, complete equation, and net ionic equation as it goes through the reaction.

http://www.slideshare.net/colinquinton/04-3144335
If you want to know more about precipitation reactions you can visit these sites
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Precipitation_Reactions
https://www.youtube.com/watch?v=IIu16dy3ThI

https://www.youtube.com/watch?v=WPnrMTCK0aU
When looking at these reactions they can be seen in the molecular equation, complete equation, and net ionic equation as it goes through the reaction.

http://www.slideshare.net/colinquinton/04-3144335
If you want to know more about precipitation reactions you can visit these sites
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Precipitation_Reactions
https://www.youtube.com/watch?v=IIu16dy3ThI
Subscribe to:
Posts (Atom)

