http://slideplayer.com/slide/4206817/
Tuesday, May 10, 2016
The ideal gas law
An ideal gas is modeled on the kinetic theory of gases which has 4 basic postulates: gases consist of small particles which are in continuous random motion, the volume of the molecules present is negligible compared to the total volume occupied by the gas, intermolecular forces are negligible, pressure is due to the gas molecules colliding with the walls of the container. The mathematical equation used to represent the ideal gas law is PV=nRT

http://slideplayer.com/slide/4206817/
http://slideplayer.com/slide/4206817/
Charles' Law
Charles' Law tells us that temperature and volume vary directly with each other. This holds true at constant pressure. Temperature for all Charles' Law problems must be in Kelvin. As the temperature of a gas increases, it gains energy. This will result in an increase in contacting the sides of their containers they are going to want to expand. The mathematical formula that represents this relationship is V1/T1=V2/T2.

http://agaul01.blogspot.com/2014/04/boyles-charles-law-in-relation-to.html
Here are some helpful links
http://www.sparknotes.com/testprep/books/sat2/chemistry/chapter5section8.rhtml
http://www.iun.edu/~cpanhd/C101webnotes/gases/charleslaw.html

http://agaul01.blogspot.com/2014/04/boyles-charles-law-in-relation-to.html
Here are some helpful links
http://www.sparknotes.com/testprep/books/sat2/chemistry/chapter5section8.rhtml
http://www.iun.edu/~cpanhd/C101webnotes/gases/charleslaw.html
Avagadro's Law
Avagadro's Law tells us that for a gas at constant temperature and pressure, the volume is directly proportional to the number of moles of gas present. Equal volumes of gases at the same temperature and pressure have the same number of particles. The mathematical formula for this relationship is V1/N1=V2/N2

http://slideplayer.com/slide/231761/
Here are some example problems

http://slideplayer.com/slide/1710660/

https://sites.google.com/site/mohshchemwithmrsp/unit-4---gas-laws/avogadro-s-law
Here are some helpful links
http://www.chemteam.info/GasLaw/Gas-Avogadro.html
http://www.britannica.com/science/Avogadros-law
https://www.youtube.com/watch?v=i-vA9uLSf7Y
http://slideplayer.com/slide/231761/
Here are some example problems
http://slideplayer.com/slide/1710660/

https://sites.google.com/site/mohshchemwithmrsp/unit-4---gas-laws/avogadro-s-law
Here are some helpful links
http://www.chemteam.info/GasLaw/Gas-Avogadro.html
http://www.britannica.com/science/Avogadros-law
https://www.youtube.com/watch?v=i-vA9uLSf7Y
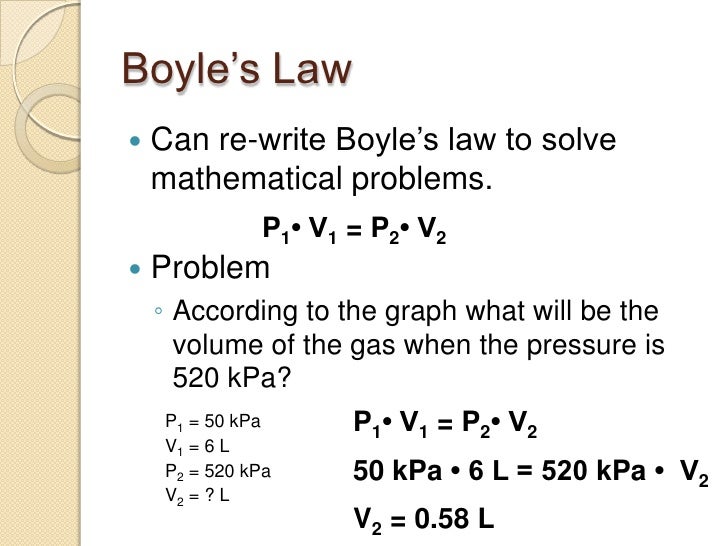
Boyle's gas law
Boyle's Law tells us that the relationship between pressure and volume is an inverse relationship. It deals with two of the four factors that determine the state of a gas: pressure and volume. It holds true at at constant temperature. The mathematical equation that is used to express this relationship is P1V1=P2V2.
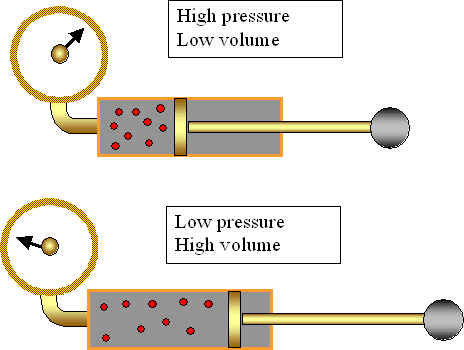
Here are some example problems

http://slideplayer.com/slide/2473563/
http://www.slideshare.net/makaberokurota/properties-of-matter-9977143
Here are some helpful websites
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/boyles-law

Here are some example problems
http://slideplayer.com/slide/2473563/

http://www.slideshare.net/makaberokurota/properties-of-matter-9977143
Here are some helpful websites
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/boyles-law
Monday, May 9, 2016
calculating heat
To calculate heat given the mass, specific heat and change in temperature we use the formula Q=MCdeltaT. this means that the heat in joules is equal to the mass X specific heat X change in temperature. We can use this formula to solve for any of the given variables even if it is not heat. For instance we can calculate the mass if given the heat in joules, specific heat, and change in temperature.
here are some ex problems

https://www.youtube.com/watch?v=txEDPJom6CU

https://www.youtube.com/watch?v=0jKHtBJNAYM
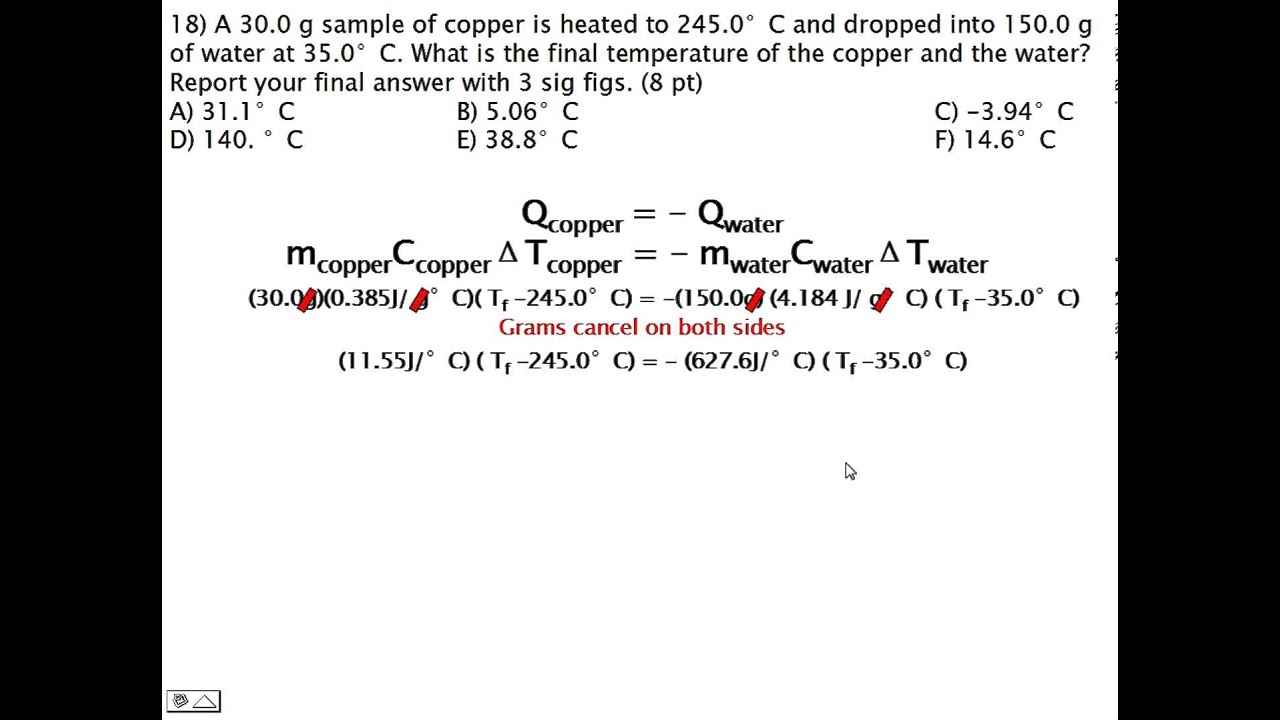
https://www.youtube.com/watch?v=EpLBZT8J3Q8
here are some more links
http://www.bbc.co.uk/schools/gcsebitesize/science/triple_aqa/calculating_energy_changes/energy_from_reactions/revision/3/
http://www.colorado.edu/physics/phys1110/phys1110_fa12/LectureNotes/Thermal.pdf
here are some ex problems

https://www.youtube.com/watch?v=txEDPJom6CU

https://www.youtube.com/watch?v=0jKHtBJNAYM

https://www.youtube.com/watch?v=EpLBZT8J3Q8
here are some more links
http://www.bbc.co.uk/schools/gcsebitesize/science/triple_aqa/calculating_energy_changes/energy_from_reactions/revision/3/
http://www.colorado.edu/physics/phys1110/phys1110_fa12/LectureNotes/Thermal.pdf
Phase cahnges and heat/cooling curves
As heat is added to a substance it undergoes some changes. Changes are changes in state which is a physical change where intermolecular bonds are broken. Melting and boiling points are determined by the vapor pressures of the solid and liquid states. At 0 degrees celsius ice and liquid water have the same vapor pressure. at 100 degrees celsius water vapor and atmospheric pressure are equal.

http://people.uwplatt.edu/~sundin/114/l114-36.htm
here are some helpful links
http://study.com/academy/lesson/what-are-heating-and-cooling-curves.html
http://www.kentchemistry.com/links/Matter/HeatingCurve.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/liquids-and-solids-11/phase-changes-90/heating-curve-for-water-394-3655/
https://www.youtube.com/watch?v=YG77v1PwQNM

http://people.uwplatt.edu/~sundin/114/l114-36.htm
here are some helpful links
http://study.com/academy/lesson/what-are-heating-and-cooling-curves.html
http://www.kentchemistry.com/links/Matter/HeatingCurve.htm
https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/liquids-and-solids-11/phase-changes-90/heating-curve-for-water-394-3655/
https://www.youtube.com/watch?v=YG77v1PwQNM
Electron dot formulas of molecules
Here are the guidelines to placing electron dot diagrams of molecules


- calculate the total number of valence electrons by adding all of the valence electrons for each atom in the molecule
- divide the total valence electrons by 2 to find the number of electron pairs in the molecule
- surround the central atom with 4 electron pair. use the remaining electron pairs to complete the octet around the other atoms
- electron pairs that are shared by atoms are called bonding electrons. the other electrons complete octets and are called non bonding electrons, or lone pairs
- if there are not enough electron pairs to provide each atom with an octet, move a non bonding electron pair between two atoms that already share an electron pair.


Covalent Bonds and Bond energy
Covalent bonds are a result of the sharing of the electrons of two nonmetal atoms. Both atoms involved in the bond share electrons to fill their particular octet. Each atom brings to the bond, the number of valence electrons and when the two combine, the octet is satisfied.
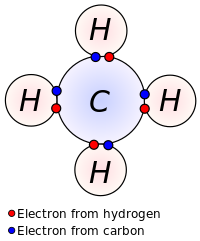
https://en.wikipedia.org/wiki/Covalent_bond
Energy is released when two ions are attracted to one another and form an ionic bond. when a bond is broken, it takes energy to do so. The energy required to break a bond is called bond energy. The amount of energy needed to form a bond is identical to the energy needed to break the bond.

http://www.science.uwaterloo.ca/~cchieh/cact/c120/bondel.html
here are some helpful websites
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Bond_Energies
https://en.wikipedia.org/wiki/Bond_energy
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/valenceframe.html

https://en.wikipedia.org/wiki/Covalent_bond
Energy is released when two ions are attracted to one another and form an ionic bond. when a bond is broken, it takes energy to do so. The energy required to break a bond is called bond energy. The amount of energy needed to form a bond is identical to the energy needed to break the bond.

http://www.science.uwaterloo.ca/~cchieh/cact/c120/bondel.html
here are some helpful websites
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Bond_Energies
https://en.wikipedia.org/wiki/Bond_energy
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/valenceframe.html
Octet rule
In atoms there are two distinct electron regions: outer shell electrons found in the s adn p blocks, and inner electrons. The octet rule will determine how many electrons are to be placed in the valence shell. This rule states that no atom can have more than 8 electrons total in the outer shell. these electrons can be shared or given over to another atoms outer shell.

https://www.youtube.com/watch?v=WzWk-mx_14E

http://chemistrytextbookcrawl.blogspot.com/2012/07/inorganic-chemistry-shriver-and-atkins_31.html
Here are some helpful sites on the rule
https://en.wikipedia.org/wiki/Octet_rule
http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Electronic_Structure_of_Atoms_and_Molecules/Electronic_Configurations/The_Octet_Rule
http://study.com/academy/lesson/understanding-ions-and-drawing-lewis-structures.html

https://www.youtube.com/watch?v=WzWk-mx_14E

http://chemistrytextbookcrawl.blogspot.com/2012/07/inorganic-chemistry-shriver-and-atkins_31.html
Here are some helpful sites on the rule
https://en.wikipedia.org/wiki/Octet_rule
http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Electronic_Structure_of_Atoms_and_Molecules/Electronic_Configurations/The_Octet_Rule
http://study.com/academy/lesson/understanding-ions-and-drawing-lewis-structures.html
Biodiesel project
For our biodiesel project we had to make a video about biodiesel and upload it to a contest for the american heart and lung associations website. For our video we decided to write a song and then sing the song in the video. The song is a parody of sweet home alabama called good ole biodiesel.
here are the lyrics

http://www.ebb-eu.org/biodiesel.php

http://biodiesel.org/

http://www.inforse.org/europe/dieret/altfuels/biodiesel.htm
here are the lyrics
You keep them big wheels turning
Carry me home to see my kin
Powering trucks across the south-land
biodiesel I’m miss’n you once again
But it’s not a sin, No
Good Ole’ Biodiesel
Keeping our skies oh so blue
Good Ole’ Biodiesel
Biodiesel, can’t get home without you
In the south-land all you need is biodeisel
It's the best renewable energy around
Well I hope you all will remember
good southern man needs biodiesel all the time
Good Ole’ Biodiesel
Keeping our skies oh so blue
Good Ole’ Biodiesel
Biodiesel, can’t get home without you
http://www.ebb-eu.org/biodiesel.php

http://biodiesel.org/

http://www.inforse.org/europe/dieret/altfuels/biodiesel.htm
What is better about biodiesel fuel
Biodiesel fuel is better for several reasons. First biodiesel is safer to hanle compared to petroleum diesel fuel, which means less risk of injury. Biodiesel is also biodegradable unlike regular petroleum fuel. Biodiesel buns clean renewable fuel and does not require modifications to diesel engines. Many alternative fuels have difficulty gaining acceptance because they do not provide similar performance to their petroleum counterparts. Pure biodiesel on the other hand provides very similar horsepower, torque, and fuel mileage compared to petroleum diesel fuel. Biodiesel emissions are also much safer and less harmful than regular petroleum emissions.

http://www.wmprocess.com/biodiesel-production/

http://abrucohk.com/Biodiesel.html
here are some links about more benefits to biodiesel
http://www.afdc.energy.gov/fuels/biodiesel_benefits.html
http://biodiesel.org/docs/ffs-basics/benefits-of-biodiesel.pdf?sfvrsn=4
http://www.berkeleybiodiesel.org/advantages-and-disadvantages-of-biodiesel.html

http://www.wmprocess.com/biodiesel-production/

http://abrucohk.com/Biodiesel.html
here are some links about more benefits to biodiesel
http://www.afdc.energy.gov/fuels/biodiesel_benefits.html
http://biodiesel.org/docs/ffs-basics/benefits-of-biodiesel.pdf?sfvrsn=4
http://www.berkeleybiodiesel.org/advantages-and-disadvantages-of-biodiesel.html
What is Biodiesel
Biodiesel is a clean burning renewable fuel made from using natural waste oils and fats. Biodiesel is is made through a chemical process which converts oils and fats of natural origin into fatty acid methyl esters. Biodiesel is intended to be used as a replacement for petroleum diesel fuel or can be blended with petroleum diesel fuel. Biodiesel is also biodegradable.

http://www.alternative-energy-news.info/technology/biofuels/biodiesel-fuel/
Here are some websites that you can find out more about biodiesel
http://www.biodiesel.com/biodiesel/what-is-biodiesel/
http://www.greenamerica.org/livinggreen/biodiesel.cfm
http://biodiesel.org/

http://www.alternative-energy-news.info/technology/biofuels/biodiesel-fuel/
Here are some websites that you can find out more about biodiesel
http://www.biodiesel.com/biodiesel/what-is-biodiesel/
http://www.greenamerica.org/livinggreen/biodiesel.cfm
http://biodiesel.org/
Thursday, March 10, 2016
Ion concentrations
In an aqueous solution of ions of a species is related to the number of males of that species per concentration of the substance in the aqueous solution.
Molarity is the number of moles of a solute divided by the total volume of the solution. M=n/v
here is an example problem
[K+]=(0.238MKNO3)×(1molK+)=0.238M
[NO−3]=(0.238MKNO3)×(1molNO−3)=0.238M
Al2(SO4)3→2Al3+(aq)+3SO2−4(aq)
[Al3+]=(0.019MAl2(SO4)3)×(2molAl3+)=0.238M
[SO2−4]=(0.019M)×(3molSO2−4)=0.057M
Molarity is the number of moles of a solute divided by the total volume of the solution. M=n/v
here is an example problem
Example 2
Determine the concentration of K+ in an aqueous solution of 0.238 M KNO3 .
SOLUTION
Since there is one mole of potassium in KNO3 , multiply the concentration of the species by the number of moles of the atom to obtain:
Although not asked, there is also one mole of nitrite ions in one mole of KNO3 , so its concentration is also 0.238 M:
FOLLOWUP
The stoichiometry always dictates the concentration, which was a simple 1:1 ratio for KNO3 . However, for more complex situations, different ratios will be encountered. For instance, if consider the dissolving of Al2(SO4)3 :
If the concentration of Al2(SO4)3 is 0.019 M, what is the concentration of Al3+(aq) ? Simply multiply 0.019 M by the stoichiometric factor of Al3+(aq) in Al2(SO4)3 , which is 2:1. The concentration of Al3+(aq) then becomes 0.038 M:
Although not asked, the concentration of SO2−4 is 0.057 M via the same argument;
[SO2−4]=(0.019M)×(3molSO2−4)=0.057M
Subscribe to:
Posts (Atom)