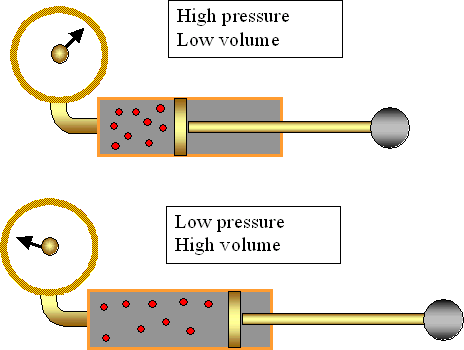
Boyle's Law tells us that the relationship between pressure and volume is an inverse relationship. It deals with two of the four factors that determine the state of a gas: pressure and volume. It holds true at at constant temperature. The mathematical equation that is used to express this relationship is P1V1=P2V2.
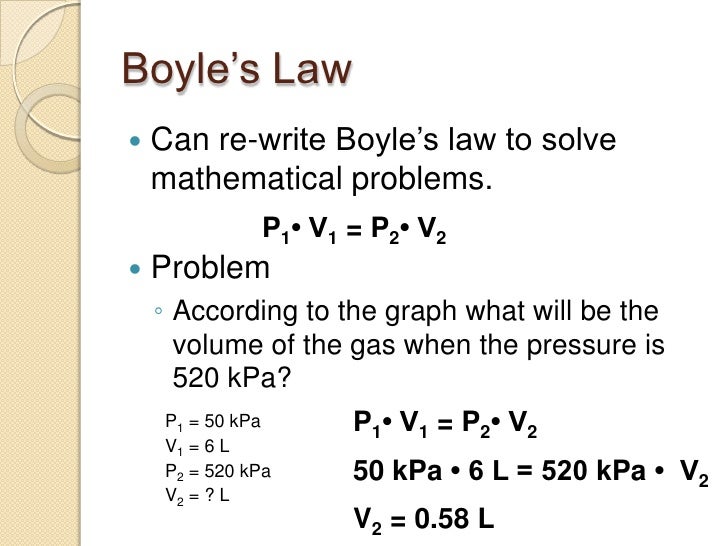
Here are some example problems
 http://slideplayer.com/slide/2473563/
http://slideplayer.com/slide/2473563/
 http://www.slideshare.net/makaberokurota/properties-of-matter-9977143
http://www.slideshare.net/makaberokurota/properties-of-matter-9977143
Here are some helpful websites
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/boyles-law


I really liked the picture representations you used here again. Being a visual learner, when I see pictures like the one you have here involving pressure and volume, it makes it easier to understand and learn.
ReplyDelete