Thursday, October 29, 2015
Post test reflection
Today we took the unit test over matter and measurement. The test overall was decently hard with some things that were more easy than others. I found that the parts over matter, and significant digits to be fairly easy. Were I had trouble was efficiently solving the conversion problems, as towards the end of the test I started to run out of time. Overall I thought that I should have done fairly well as I studied quite a bit the night before.
Significant figure rules
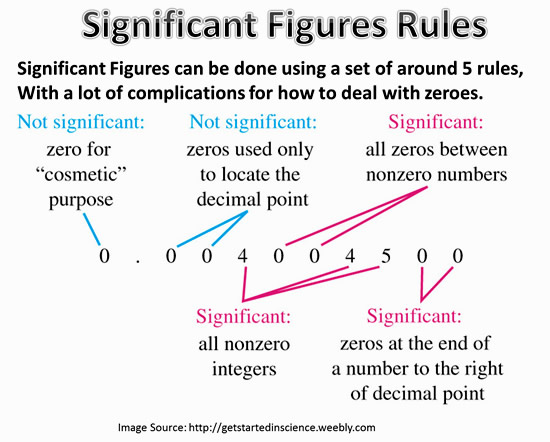
In this unit we covered a concept called significant figures. Significant figures are each of the digits of a number that are used to express it to the required degree of accuracy, starting from the first nonzero digit.
http://passyworldofmathematics.com/significant-figures/
There are several rules that go along with significant figures which can be seen above in the picture. The first rule is that all non-zero numbers are always significant. The second rule is that all zeros between non-zero numbers are always significant. The third rule is that all zeros which are simultaneously to the right of the decimal point and at the end of the number are always significant. The last rule is that all zeros which are to the left of the written decimal point are in a number greater than ten are always significant.
http://www.usca.edu/chemistry/genchem/sigfig.htm

http://passyworldofmathematics.com/significant-figures/
There are several rules that go along with significant figures which can be seen above in the picture. The first rule is that all non-zero numbers are always significant. The second rule is that all zeros between non-zero numbers are always significant. The third rule is that all zeros which are simultaneously to the right of the decimal point and at the end of the number are always significant. The last rule is that all zeros which are to the left of the written decimal point are in a number greater than ten are always significant.
| Number | # Significant Figures | Rule(s) |
| 48,923 | 5 | 1 |
| 3.967 | 4 | 1 |
| 900.06 | 5 | 1,2,4 |
| 0.0004 (= 4 E-4) | 1 | 1,4 |
| 8.1000 | 5 | 1,3 |
| 501.040 | 6 | 1,2,3,4 |
| 3,000,000 (= 3 E+6) | 1 | 1 |
| 10.0 (= 1.00 E+1) | 3 | 1,3,4 |
Here are some helpful links to learn more
Sunday, October 25, 2015
Matter
In our first section of measurement we covered matter. Matter is anything that has mass and takes up space. There are three states in which we find matter, which are water, liquid, and gas. We classify this matter using a chart with many levels. if the matter is uniform it is homogeneous. If the matter is not then it is heterogeneous. If the matter was homogeneous and has a variable composition it is a homogeneous mixture, but if it doesn't have a variable composition then it is a pure substance. If the matter is a pure substance and can be separated into simple substances it is a compound, and if not it is an element. With matter there are physical properties which are observed without changing into another substance, and there are chemical properties which can only be observed when changing into another substance. Some physical properties are boiling point,and density. Some chemical properties are heat of combustion, chemical stability, and toxicity.

http://sciencewithme.com/learn-about-matter/

https://www.pinterest.com/pin/478085316665314945/
here are some more helpful links about matter.
https://en.wikipedia.org/wiki/Mixture
http://www.ducksters.com/science/chemistry/chemical_mixtures.php
http://www.chem4kids.com/files/matter_states.html

http://sciencewithme.com/learn-about-matter/

https://www.pinterest.com/pin/478085316665314945/
here are some more helpful links about matter.
https://en.wikipedia.org/wiki/Mixture
http://www.ducksters.com/science/chemistry/chemical_mixtures.php
http://www.chem4kids.com/files/matter_states.html
Mole Day
On Friday we celebrated mole day in class. Mole day is an unofficial holiday celebrated among chemists on October 23, which celebrates Avogadro's number 6.02 times 10^23. To celebrate mole day we had to make a mole out of fabric and sew it together to bring into class. At first I wasn't sure how I was going to make the mole, because I didn't think I would be able to sew, but it wasn't as hard as I thought it was going to be. The first step to making the mole was to cut out the fabric to match the templates that our teacher gave us. After I had all of these cut out i started to sew along the lines as the directions stated. I then stuffed the mole, finished sewing, and then finally added eyes, nose, and a tail. I thought that this project would be fairly difficult but it turned out not to be that bad.
Thursday, October 1, 2015
Decay
In this Unit we learned about three different types of decay, alpha, beta, and gamma. Many nuclei are radioactive, which means they spontaneously decay, forming a different nucleus and producing one or more particles. The first type of decay we covered was alpha decay. Alpha decay is a common mode for heavy radioactive nuclides resulting in a loss of 4 in the mass number and a loss of 2 in atomic number.
Here is an example of Alpha decay

http://www.chemteam.info/Radioactivity/Writing-Alpha-Beta.html
The second type of decay we learned about was beta decay. Beta decay is a decay process for radioactive nuclides in which mass number does not change and the atomic number increases by 1.
Here is an example of Beta decay
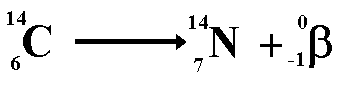
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
The third and final type of decay type of decay we learned about was Gamma decay. In gamma decay a nucleus changes from a higher energy state to a lower energy state through the emission of electromagnetic radiation but does not change the mass number, or the proton number.
Here is an example of Gamma decay
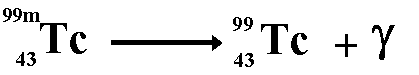
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
If you are wishing to learn more about these types of decay you can go to these websites
Alpha-http://education.jlab.org/glossary/alphadecay.html
Beta-https://en.wikipedia.org/wiki/Beta_decay
Gamma-http://www2.lbl.gov/abc/wallchart/chapters/03/3.html
Here is an example of Alpha decay
http://www.chemteam.info/Radioactivity/Writing-Alpha-Beta.html
The second type of decay we learned about was beta decay. Beta decay is a decay process for radioactive nuclides in which mass number does not change and the atomic number increases by 1.
Here is an example of Beta decay
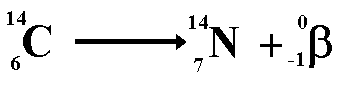
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
The third and final type of decay type of decay we learned about was Gamma decay. In gamma decay a nucleus changes from a higher energy state to a lower energy state through the emission of electromagnetic radiation but does not change the mass number, or the proton number.
Here is an example of Gamma decay
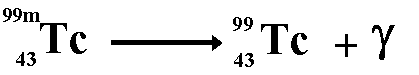
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
If you are wishing to learn more about these types of decay you can go to these websites
Alpha-http://education.jlab.org/glossary/alphadecay.html
Beta-https://en.wikipedia.org/wiki/Beta_decay
Gamma-http://www2.lbl.gov/abc/wallchart/chapters/03/3.html
Forensic Archaeology lab
The archaeology lab started with getting a piece of paper which had two sides. On one side of the paper it had color, and on the other it was plain white. The first step in the lab was to cut the piece of paper into 567 squares. Each of these squares would have a white side and a colored side. after we had all of the squares cut out we put them into a cup and dumped them out. we divided the squares into the ones that had the colored side face up and the white side face up.the atoms that were colored side up represented the atoms that had decayed and were taken out from the rest of the pieces. we repeated the process of dumping and removing the decayed squares for 6 times. this process represents half life of a substance as it decays.
If you want to learn more about half life visit these website
Subscribe to:
Posts (Atom)


