A few days ago on Friday we took the aqueous solutions test. I felt that this test was very similar to what we had been doing on the review guides and didn't seem like there was too much information that I didn't know on the test.
here are some of the links that I used to study for the test
http://dl.clackamas.edu/ch105-04/calculat.htm
http://www.occc.edu/kmbailey/Chem1115Tutorials/Molarity.htm
http://www.chemteam.info/Solutions/Molarity.html
Sunday, January 24, 2016
Aqueous solutions basics
When the components of a mixture are uniformly intermingled- that is the mixture is homogeneous in nature- it is called a solution. There are various types of solutions and here is a list of some.

although a lot of substances can dissolve in water there are many that are insoluble in water. most of those substances are non polar in nature. non polar substances have elements in them have similar electronegatives and those elements are symmetrically distributed in the compound.
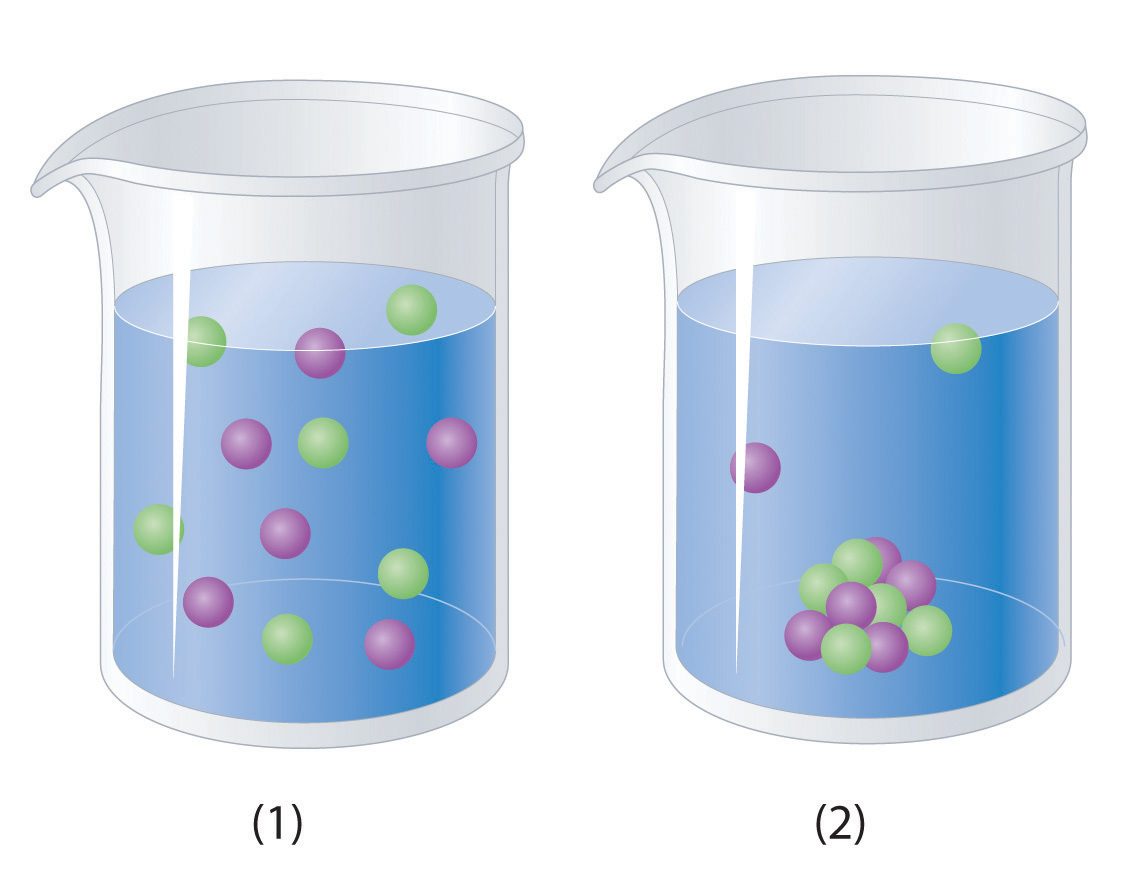
here are some helpful links for aqueous solutions.
http://chemistry.bd.psu.edu/jircitano/aqueous.html
http://chemwiki.ucdavis.edu/Wikitexts/United_Arab_Emirates_University/UAEU_CHEM_111/Full_Chapters/Full_Chapter_04._Reactions_in_Aqueous_Solution
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Unique_Features_of_Aqueous_Solutions

although a lot of substances can dissolve in water there are many that are insoluble in water. most of those substances are non polar in nature. non polar substances have elements in them have similar electronegatives and those elements are symmetrically distributed in the compound.

here are some helpful links for aqueous solutions.
http://chemistry.bd.psu.edu/jircitano/aqueous.html
http://chemwiki.ucdavis.edu/Wikitexts/United_Arab_Emirates_University/UAEU_CHEM_111/Full_Chapters/Full_Chapter_04._Reactions_in_Aqueous_Solution
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Unique_Features_of_Aqueous_Solutions
murder investigation lab
The purpose of the murder investigation lab was to use molarity and stoichiometry to determine the unknown substance.Then using the information from the lab to determine the murderer.
Here were the steps we took to perform the lab
Here were the steps we took to perform the lab
Procedure:
- weigh piece piece of filter paper
- fold the filter paper so that it can fit into a funnel
- place the filter paper and funnel into a beaker
- obtain 10 ml of unknown solution and 28 ml of NaCl
- pour both the unknown solution and the NaCl into a beaker that can hold 80ml
- pour the beaker of mixed solution into the funnel with the funnel paper
- allow for all of the liquid to filter through the filter paper
- after all of the liquid has gone into the beaker remove the filter paper and allow the filter paper to dry
- weigh filter paper to get the amount of precipitate formed
Subscribe to:
Posts (Atom)

