This unit test didn't go as well as I expected it to go. On the quiz for this unit I did very well, and I thought that I was well prepared for this test. The things I think I messed up on were the ice box problems and some of the problems that were mostly definition. All in all the unit was not too bad, I just wish that I could have done better on the test.
Here are some things i used to study.
http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Equilibria/Le_Chatelier's_Principle/Ice_Tables
http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Acids_and_Bases/Acid%2F%2FBase_Reactions/Conjugate_Acids-base_Pairs
https://www.khanacademy.org/science/chemistry/acids-and-bases-topic/copy-of-acid-base-equilibria/v/conjugate-acids-and-bases
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch11/conjugat.php
Sunday, February 28, 2016
pH and pOH conversion square
To determine the pH of a solution or the pOH of a solution, we need to know the (H+) concentration or the (OH-) concentration. Using these we can calculate the pH, pOH, and the H+ if given the OH-, and the OH- if given the H+.
Here is the square that we use to go from one concentration to another.

http://www.fullerchemistry.com/Acid-Base%20Chemistry/acid.htm
Here is the square that we use to go from one concentration to another.
http://www.fullerchemistry.com/Acid-Base%20Chemistry/acid.htm
The H+ Ion in water
The H+ ion is simply a proton (nucleus of a hydrogen atom without its valence electron)
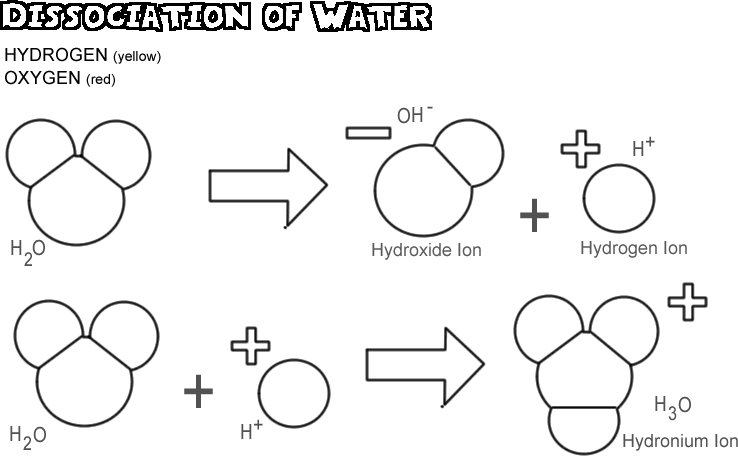
- In water, clusters of hydrated H+ ions form
- The simplest cluster is H3O+
- we call this the hydronim ion
- Larger clusters are also possible (such as H5O2 and H9O4)
Generally we use H+ and H3O+ interchangeably

Bronsted-Lowery Acids and Bases
In the B.L. definitions, we have conjugate acid and conjugate bases. These come in pairs.
Acids produce conjugate bases and bases produce conjugate acids
- Conjugate acid: the substance formed when a proton is added to a base
- Conjugate base: the remaining substance when a proton is lost from an acid

http://tanmayonrun.blogspot.com/2011/10/what-do-you-mean-by-conjugate-acid-base.html
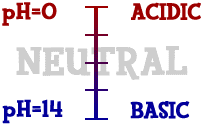
Acid and Base basics
Acids and bases have distinct physical properties
http://www.chem4kids.com/files/react_acidbase.html
- Acids:taste sour and feel sticky
- Bases:taste bitter and feel slippery
- There are several different definitions of acids and bases and it is important to distinguish between them all
- Bases=7.1-14
- acids=1-6.9
- Arrhenius acids are those species that produce hydrogen ions in solution
- Arrhenius bases are those species that produce hydroxide ions in solution

http://www.chem4kids.com/files/react_acidbase.html
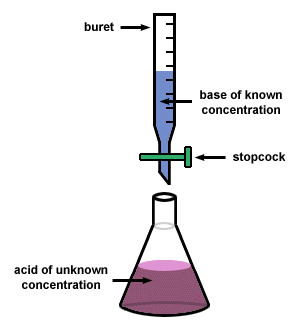
Percent Acetic Acid in Vnegar Lab
In this lab, we standardized a solution of NaOH with potassium hydrogen phthalate in a titration to determine the molarity of NaOH solution. We then used this standard NaOH solution to determine the % acetic acid in commercial vinegar.
Here were the steps to the titration.\
http://www.sparknotes.com/chemistry/acidsbases/titrations/section1.rhtml
Here were the steps to the titration.\

http://www.sparknotes.com/chemistry/acidsbases/titrations/section1.rhtml
- Put on splash proof safety glasses
- drain buret into the waste beaker
- rinse your buret with a small amount of NaOH
- with the valve closed, add NaOH until it is above the zero mark
- Obtain 0.5-0.8 g of KHP
- transfer KHO into Erlenmeyer flask
- add 75ml of water to flask
- add 2-3 drops of Phenolphthalein
- open vial with blue cap
- pipet 10ml of vinegar into flask
- drain buret, fill with distilled water
Subscribe to:
Posts (Atom)