Here is an example of Alpha decay
http://www.chemteam.info/Radioactivity/Writing-Alpha-Beta.html
The second type of decay we learned about was beta decay. Beta decay is a decay process for radioactive nuclides in which mass number does not change and the atomic number increases by 1.
Here is an example of Beta decay
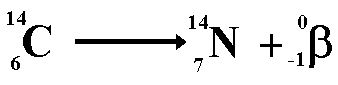
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
The third and final type of decay type of decay we learned about was Gamma decay. In gamma decay a nucleus changes from a higher energy state to a lower energy state through the emission of electromagnetic radiation but does not change the mass number, or the proton number.
Here is an example of Gamma decay
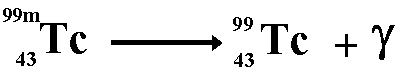
http://www.cyberphysics.co.uk/topics/radioact/Radio/equations.htm
If you are wishing to learn more about these types of decay you can go to these websites
Alpha-http://education.jlab.org/glossary/alphadecay.html
Beta-https://en.wikipedia.org/wiki/Beta_decay
Gamma-http://www2.lbl.gov/abc/wallchart/chapters/03/3.html
The pictures were a very good visual way of describing the different types of radioactive decay.
ReplyDeleteThe pictures were a very good visual way of describing the different types of radioactive decay.
ReplyDeleteThe pictures explain each type of radioactive decay very well.
ReplyDelete